Key Market Insights
- Over 175 companies claim to offer fill / finish services for various types of biopharmaceutical drug products; the growth initiatives, in this segment, are being led by large and mid-sized players
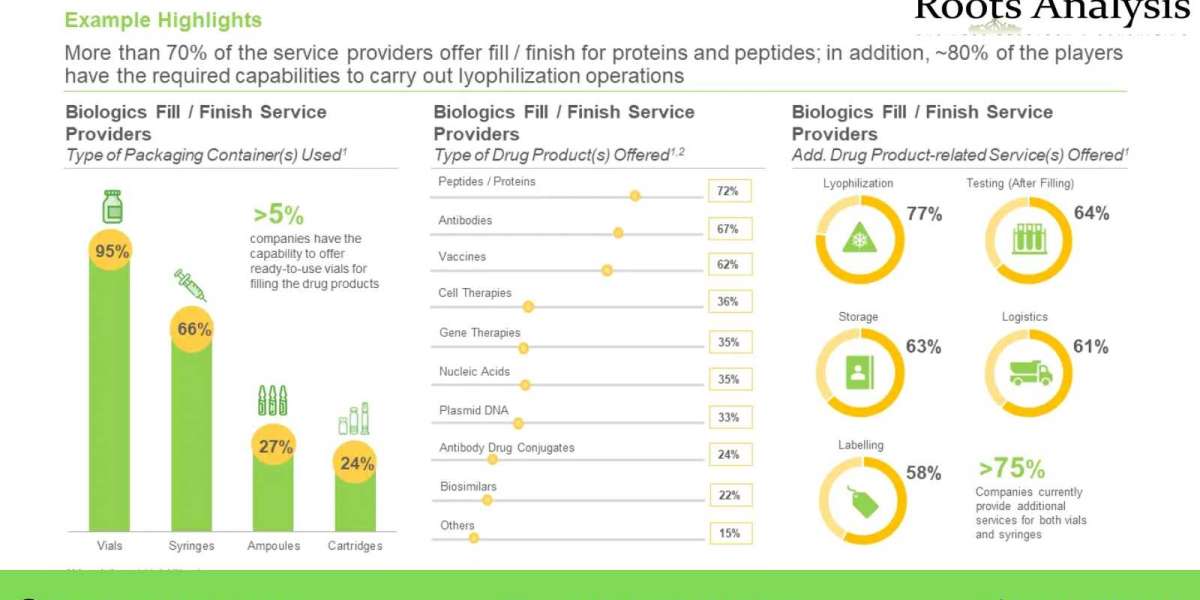
- More than 70% of the service providers offer fill / finish for proteins and peptides; in addition, ~80% of the players have the required capabilities to carry out lyophilization operations
- Around 125 industry players offer fill / finish services for various types of small molecule drug products, across different scales of operation
- Leveraging their expertise, 85% stakeholders extend services for labelling and packaging, both for clinical and commercial scale operations
- In order to expand their aseptic fill / finish service portfolios and gain access to emerging technologies, companies are actively entering into strategic partnerships
- The aseptic fill finish market is likely to grow at a CAGR of ~11%, till 2035; this opportunity is expected to be well distributed across different types of molecules, packaging containers and drug products
- In the long-term, the US (~30%) is likely to significantly contribute towards the market growth, followed by Asia-Pacific countries; specifically, within Europe, Germany and France are like to account for ~5% market share, each
Table of Contents
TABLE OF CONTENTS
- PREFACE
1.1. Chapter Overview
1.2. Key Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Introduction to Contract Manufacturing
3.3. Commonly Outsourced Operations in Biopharmaceutical Industry
3.4. Basic Guidelines for Selecting a Fill and Finish Service Provider
3.5. Advantages of Outsourcing Fill / Finish Operations
3.6. Risks and Challenges Associated with Outsourcing Fill / Finish Operations
3.7. Concluding Remarks
- ASEPTIC FILL AND FINISH SERVICE PROVIDERS LANDSCAPE
4.1. Chapter Overview
4.2. Aseptic Fill / Finish: Service Providers Landscape
- BIOLOGICS FILL / FINISH SERVICE PROVIDERS LANDSCAPE
5.1. Chapter Overview
5.2. Biologics Fill / Finish: Service Providers Landscape
- SMALL MOLECULE FILL / FINISH SERVICE PROVIDERS LANDSCAPE
6.1. Chapter Overview
6.2. Small Molecule Fill / Finish: Service Providers Landscape
- COMPANY PROFILES: ASEPTIC FILL / FINISH SERVICE PROVIDERS IN NORTH AMERICA
7.1. Chapter Overview
7.2. Companies Offering Aseptic Fill / Finish Services for Biologics
7.2.1. AbbVie Contract Manufacturing
7.2.1.1. Company Snapshot
7.2.1.2. Financial Information
7.2.1.3. Service Portfolio
7.2.1.4. Fill / Finish Services Offered
7.2.1.5. Recent Developments and Future Outlook
7.2.2. BioPharma Solutions
7.2.3. BioReliance
7.2.4. Catalent Biologics
7.2.5. Charles River Laboratories
7.2.6. Patheon
7.3. Companies Offering Aseptic Fill / Finish Services for Small Molecules
7.3.1.1. Pfizer CentreOne
7.3.1.2. Company Snapshot
7.3.1.3. Service Portfolio
7.3.1.4. Fill / Finish Services Offered
7.3.1.5. Recent Developments and Future Outlook
7.3.2. Plastikon Healthcare
7.3.3. Sharp Services
- COMPANY PROFILES: ASEPTIC FILL / FINISH SERVICE PROVIDERS IN EUROPE
8.1. Company Overview
8.2. Companies Offering Aseptic Fill / Finish Services for Biologics
8.2.1. Boehringer Ingelheim BioXcellence
8.2.1.1. Company Snapshot
8.2.1.2. Financial Information
8.2.1.3. Service Portfolio
8.2.1.4. Fill / Finish Services Offered
8.2.1.5. Recent Developments and Future Outlook
8.2.2. Fareva
8.2.3. GlaxoSmithKline
8.2.4. Lonza
8.2.5. Pierre Fabre
8.2.6. Wacker Biotech
8.3. Companies Offering Aseptic Fill / Finish Services for Small Molecules
8.3.1. Aenova
8.3.2. APL
8.3.3 CordenPharma
8.3.4. Delpharm
8.3.5 PiSA Farmaceutica
8.4 Companies Offering Aseptic Fill / Finish Services for Biologics and Small Molecules
8.4.1 Fresenius Kabi
8.4.2 Recipharm
- COMPANY PROFILES: ASEPTIC FILL / FINISH SERVICE PROVIDERS IN ASIA-PACIFIC
9.1 Company Overview
9.2 Companies Offering Aseptic Fill / Finish Services for Biologics
9.2.1 Asymchem
9.2.2. Samsung Biologics
9.2.3. Syngene
9.2.4. Takara Bio
9.2.5. WuXi Biologics
9.2.6. Hetero Drugs
9.2.7. Intas Pharmaceuticals
9.3. Companies Offering Aseptic Fill / Finish Services for Small Molecules
9.3.1. Siegfried
9.3.2. Wockhardt
- PARTNERSHIPS AND COLLABORATIONS
10.1. Chapter Overview
10.2. Partnership Models
10.3. Aseptic Fill / Finish Service Providers: List of Partnerships and Collaborations
- MARKET SIZING AND OPPORTUNITY ANALYSIS
11.1. Chapter Overview
11.2. Forecast Methodology
11.3. Overall Aseptic Fill / Finish Services Market, 2023-2035
11.4. Aseptic Fill / Finish Services Market for Biologics, 2023-2035
11.5. Aseptic Fill / Finish Services Market for Small Molecules, 2023-2035
- CASE STUDY: ROBOTICS IN PHARMACEUTICAL PACKAGING
12.1. Chapter Overview
12.2. Role of Robotic Systems in Pharmaceutical Industry
12.3. Role of Robotic Systems in Fill / Finish Operation
12.4. Contract Service Providers: List of Fill / Finish Equipment
12.5. Companies Providing Robots for Use in the Pharmaceutical Industry
12.6. Companies Providing Isolator based Aseptic Filling Systems
12.7. Concluding Remarks
- CASE STUDY: READY-TO-USE PACKAGING COMPONENTS FOR ASEPTIC FILL / FINISH
13.1. Chapter Overview
13.2. Role of Ready-to-Use Packaging Components in Aseptic Fill / Finish Operations
13.3. Companies Providing Ready-to-Use Packaging Components
13.4. Concluding Remarks
- CONCLUDING REMARKS
- EXECUTIVE INSIGHTS
15.1 Chapter Overview
15.2 IDT Biologika
15.3 Cytovance Biologics
15.4. Syngene
15.5 oncomed manufacturing
15.6 Yposkesi
15.7 HALIX
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/aseptic-fill-finish-market.html
Learn from experts: do you know about these emerging industry trends?
Liposomes Development: Service Providers Landscape
mRNA Synthesis: Manufacturing Process of Modern Revolutionary Molecule
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415