Global Duchenne Muscular Dystrophy Drugs Industry: Key Statistics and Insights in 2024-2032
Summary:
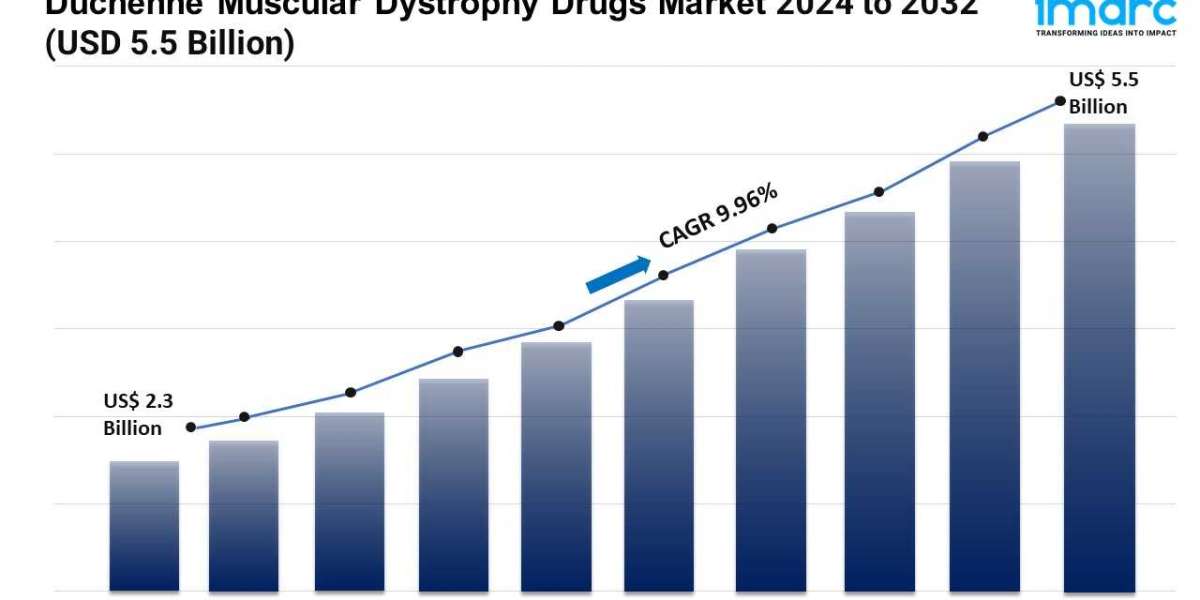
- The global duchenne muscular dystrophy drugs market size reached USD 2.3 Billion in 2023.
- The market is expected to reach USD 5.5 Billion by 2032, exhibiting a growth rate (CAGR) of 9.96% during 2024-2032.
- North America's leads the market, accounting for the largest duchenne muscular dystrophy drugs market share.
- Governing agencies worldwide are offering incentives to encourage the development of treatments for rare diseases like DMD.
- These incentives include tax credits, user fee waivers, and market exclusivity to make it economically viable for drug companies to invest in conditions with relatively small patient populations.
- Such support is crucial in driving the development and rapid approval of new drugs, as it offsets the high costs and risks associated with pharmaceutical development in rare diseases.
- Increased healthcare spending and the willingness of insurance companies to cover high-cost treatments for rare diseases contribute to market growth.
- These factors together create a favorable environment for the development and commercialization of treatments for rare diseases like DMD.

Grab a sample PDF of this report: https://www.imarcgroup.com/duchenne-muscular-dystrophy-drugs-market/requestsample
Industry Trends and Drivers:
- Increasing Disease Prevalence:
The escalating demand for Duchenne muscular dystrophy (DMD) drugs due to the rising prevalence of DMD is propelling the market growth. Moreover, the wide accessibility of genetic testing, along with the increasing awareness among healthcare providers about the symptoms and genetic markers of DMD, is impelling the market growth. Early diagnosis is crucial for the management of DMD, leading to a higher demand for therapeutic solutions. This trend is observed worldwide, not just in developed countries but also in regions where genetic testing and healthcare infrastructure are improving.
- Technological Advancements in Treatment:
Innovations in gene therapy and molecular medicine are opening new opportunities for DMD treatment. Techniques, such as CRISPR gene editing and exon skipping, allow for the correction of genetic anomalies that cause DMD at a molecular level. These advanced therapies, although in varying stages of research and trial, promise potentially transformative improvements in patient outcomes. These technologies not only drive investment from pharmaceutical companies but also gain interest from regulatory bodies and investors, accelerating the pace of research and trials aimed at finding a cure or more effective treatments for DMD.
- Advancements in Drug Delivery Systems:
In DMD, patients may have limitations related to muscle weakness and damage, innovative drug delivery technologies that are less invasive and more efficient can make a difference. The development of sustained-release formulations, transdermal patches, or nanoparticle-based delivery systems can improve how drugs are administered, enhancing their absorption while reducing side effects. In line with this, improved drug delivery systems are therefore a critical driver, making treatment options more tolerable and potentially effective, thereby increasing their adoption and market expansion worldwide.
The duchenne muscular dystrophy drugs market report provides a comprehensive overview of the industry. This analysis is essential for stakeholders aiming to navigate the complexities of the biochar market and capitalize on emerging opportunities.
Duchenne Muscular Dystrophy Drugs Market Report Segmentation:
By Therapeutic Approach:

- Mutation Suppression
- Exon Skipping
- Steroid Therapy
Exon skipping holds the biggest market share due to the rising focus on targeted gene therapy.
By End User:
- Hospitals
- Clinics
- Home Care Settings
Hospitals account for the largest market share, driven by access to advanced treatment solutions.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys a leading position in the Duchenne muscular dystrophy drugs market on account of the presence of advanced healthcare systems.
Top Duchenne Muscular Dystrophy Drugs Market Leaders:
The duchenne muscular dystrophy drugs market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- FibroGen Inc.
- Italfarmaco S.p.A.
- NS Pharma Inc. (Nippon Shinyaku Co. Ltd.)
- PTC Therapeutics Inc.
- Santhera Pharmaceuticals
- Sarepta Therapeutics Inc.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145